- Messages
- 3,482
- Location
- Essex, UK
I'm opening this up for a discussion and research really.
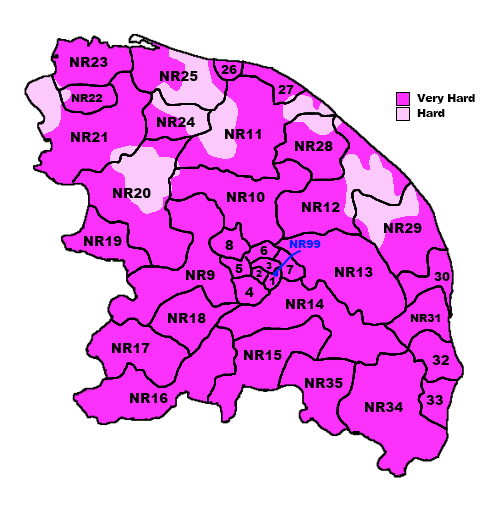
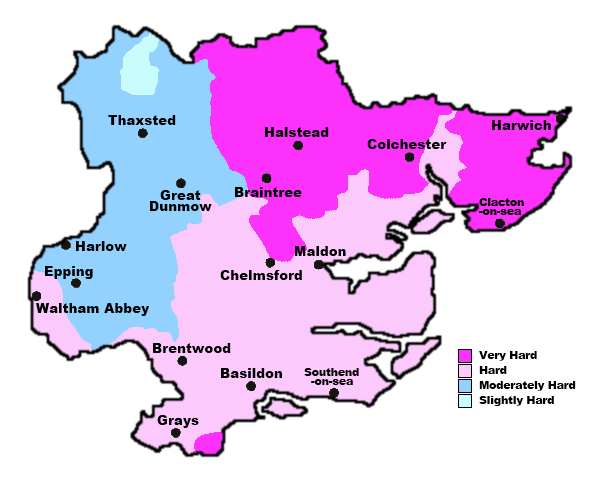
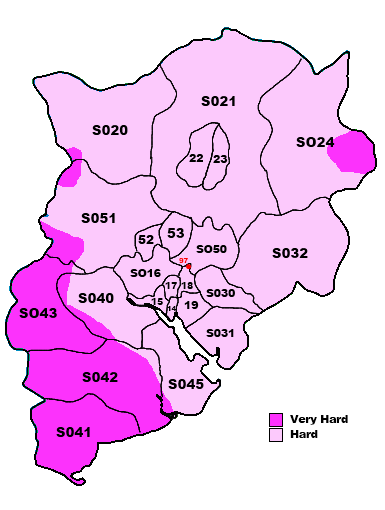
What do we think we know, hard water makes it harder work getting a decent lather. We have Norfolk residents who have abandoned MWF because of it. We have Linconshire residents needing a separate bowl to generate lather.
But then we have me, who also has hard water, being a brewer I know my water's chemistry intimately, I can lather MWF with a quick (30 second) swirl of the puck, then onto my face for a face lather.
Two possibilites.
1. Its just technique and I'm better. Highly unlikely.
2. Its not hardness at all that matters, but something else. Could it be the Alkalinity of the water, or some other mineral content entirely.
Anyone any thoughts. I'd like to understand this as I keep seeing posts from people saying its the fault of their hard water that they can't lather x and I think, but I have hard water and it works fine for me. MWF is my favourite soap.
What do we think we know, hard water makes it harder work getting a decent lather. We have Norfolk residents who have abandoned MWF because of it. We have Linconshire residents needing a separate bowl to generate lather.
But then we have me, who also has hard water, being a brewer I know my water's chemistry intimately, I can lather MWF with a quick (30 second) swirl of the puck, then onto my face for a face lather.
Two possibilites.
1. Its just technique and I'm better. Highly unlikely.
2. Its not hardness at all that matters, but something else. Could it be the Alkalinity of the water, or some other mineral content entirely.
Anyone any thoughts. I'd like to understand this as I keep seeing posts from people saying its the fault of their hard water that they can't lather x and I think, but I have hard water and it works fine for me. MWF is my favourite soap.